How to Identify Catalyst in Reaction
Identifying CombinationSynthesis Reactions Download Article 1. 3 Page 10 of 35 b Ethanenitrile can be made by reacting chloromethane with potassium cyanide.

Practice Identifying Catalysts And Intermediates Youtube
A slurry of polyethylene unreacted ethylene monomer catalyst and solvent exit the reactor.

. 76 Describe a catalyst as a substance that speeds up the rate of a reaction without altering the products of the reaction being itself unchanged chemically and in mass at the end of the reaction. Write formulas for the following compound. Enzymes are naturally occurring catalysts responsible for many essential biochemical reactions.
4d the peaks at 1060 and 982 cm 1 represent HSO 5-and SO 4 2- respectively Zhang et al 2013. Dynamic oxygen adsorption on single-atomic Ruthenium catalyst with high performance for acidic oxygen evolution reaction. Enzyme kinetics is the study of the rates of enzyme-catalysed chemical reactionsIn enzyme kinetics the reaction rate is measured and the effects of varying the conditions of the reaction are investigated.
Knowing the properties of each reaction will help you identify each one. 78 Recall that enzymes are biological catalysts and that enzymes are used in the production of alcoholic drinks. The guide covers all the necessary reactions from the beginning of Org 1 Structure and Bonding to the end of Org 2 Amino Acids and everything in.
The chemical nature of the reacting substances the state of subdivision one large lump versus many small particles of the reactants the temperature of the reactants the concentration of the reactants and the presence of a catalyst. A The rate of a chemical reaction changes with time. C The rate of a chemical reaction is affected by the temperature of the reaction.
Here the CuCeO 2 catalyst as paradigm was studied to validate the reaction intermediates and pathway. Two hydrogen atoms are added to the. Where it polymerizes under the influence of a Ziegler-Natta catalyst in the presence of a solvent.
Unreacted ethylene is separated and returned to the reactor while the catalyst is neutralized by. In case of unsymmetrical alkynes addition of water occurs according to Morkovnikvs rule. Ii Identify a catalyst used in a catalytic converter.
Nat Commun 10 4849 2019. A combinationsynthesis reaction is aptly named because it is a reaction where 2 or more products combine together to form 1 new product. Method 1 of 6.
Write formulas for the. Can you identify which type of chemical reaction is shown. Count the number of reactants.
Chemical reaction in which the products reform the original reactants. Catalyst in chemistry any substance that increases the rate of a reaction without itself being consumed. Insights into the role of dual reaction sites for single Ni atom Fenton-like catalyst towards degradation of various organic contaminants.
It does not participate in the chemical reaction. Studying an enzymes kinetics in this way can reveal the catalytic mechanism of this enzyme its role in metabolism how its activity is controlled and how a drug or a modifier. Identify the INCORRECT statement below concerning chemical kinetics.
Topic 7 - Rates of. Products of alkyne with HgSO 4 and H 2 SO 4 reaction. Therefore the ratio of I 1060 I.
B The rate constant of a reaction generally depends on the concentrations of species. The CuCeO 2 catalysts with different morphologies were synthesized and it is. Identify the main organic product when a large excess of chlorine is used in this reaction.
As shown in Fig. Most solid catalysts are metals or the oxides sulfides and halides of metallic elements and of the semimetallic elements boron aluminum and silicon. Operation of a three-way catalyst.
Chem 1120 - Chapter 13. A three-way catalyst oxidizes exhaust gas pollutants - both hydrocarbons HC and carbon monoxide CO - and reduces nitrogen oxides NO x into the harmless components water H 2 O nitrogen N 2 and carbon dioxide CO 2Depending on the operating conditions of the engine and the exhaust gas composition conversion rates upwards. The determination of reaction mechanism for electrocatalytic CO 2 reduction by experiments is still out of reach on copper catalyst which limits its advance towards industrial implementation.
The rate of a reaction depends. We can identify five factors that affect the rates of chemical reactions. Further substitution can occur during this reaction.
I developed the Organic Chemistry Reaction and Mechanism Guide to help you understand more than 185 of the most common reactions encountered in undergraduate organic chemistry. The Chemical Nature of the Reacting Substances. Substance that changes the rate of a chemical reaction but can be recovered unchanged.
Therefore we use HgSO 4 as the catalyst to increase the reaction rate. To improve catalytic activity and stability and to mitigate the current need to use a high reaction temperature a rational bottom-up catalyst. Rates of Reaction Practice Quiz 1.
Test your knowledge with this quiz. I Write an equation for this. Noun a substance that enables a chemical reaction to proceed at a usually faster rate or under different conditions as at a lower temperature than otherwise possible.
To further identify the type of nonradical pathway the in situ Raman was performed. Cao L Luo Q Chen J. Oxygen atom is added to one carbon atom in the triple bond and two hydrogen atoms are added to other carbon atom.
D The rate law.

Is It A Catalyst Or An Intermediate Given Mechanism Youtube

How To Identify The Intermediate Catalyst In A Reaction Mechanism Kinetics Chemistry Youtube
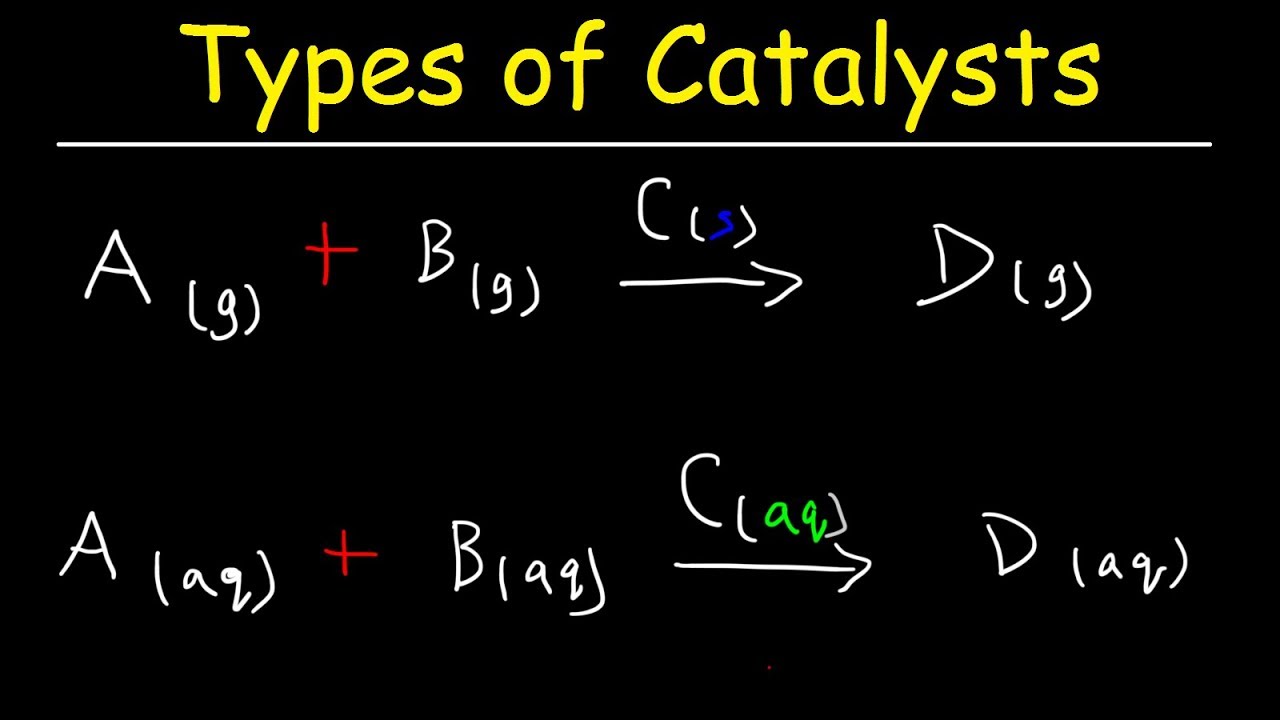
How To Identify The Intermediate Catalyst In A Reaction Mechanism Kinetics Chemistry Youtube
0 Response to "How to Identify Catalyst in Reaction"
Post a Comment